|
|
| ||
|---|---|---|---|
 | 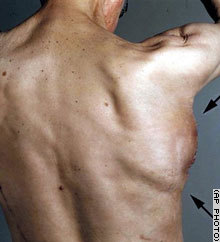 | ||
| Worcester, 1996 | Kiel, 2004
| ||
But is this really progress? CNN is reporting today:
According to this week's issue of The Lancet medical journal, the German doctors used a mesh cage, a growth chemical and the patient's own bone marrow, containing stem cells, to create a new jaw bone that fit exactly into the gap left by the cancer surgery.
Tests have not been done yet to verify whether the bone was created by the blank-slate stem cells and it is too early to tell whether the jaw will function normally in the long term.
But the operation is the first published report of a whole bone being engineered and incubated inside a patient's body and transplanted.
I've made some small contributions to the study of cell attachment and mechanotransduction, with an eye toward enhancing applications like this. But having looked at Warnke's Lancet paper (subscription required), I'm a little disturbed. This research doesn't seem based on any hypothesis, practicality, or need.
As far as I can tell, the surgeons could've designed and built a well-fitting, complete titanium jaw and secured it with established, titanium osteosynthesis screws. This could've been done in one operation, with familiar and established risks.
Instead, here's what they did: they designed and built a titanium mesh, filled it with carrier bone mineral blocks, coated with a recombinant human bone growth factor embedded in cow collagen, squirted in some of the patient's bone marrow to theoretically supply stem cells for future bone growth, implanted it in his lat, waited 7 weeks, confirmed some bony synthesis with a radioactive scan, took it out, and put it in his face. They chose a course of at least three operations, involving cow parts, high doses of expensive growth factors, radiation, and unknown tissue products, with a plan to remove the original titanium scaffold in a year, and see if what's left can support force. The authors cite "ethical concerns" for not histologically sampling the tissue to see what, exactly, they're putting in the guy's mouth after weeks of incubation on his back. Is it bone? Is it mostly scar tissue? Is it osteosarcoma? Is the cow tissue causing inflammation? None of these questions were examined.
(There's some handwaving in the piece about how the patient could not have accepted an autologous graft, because his coumadin predisposes bleeding from potential donor sites. There's additional reports that the patient was miserable with his old, subtotal titantium implant, and CNN adds that total artificial implants risk infection. I don't see how the alternative -- titanium mesh, bovine collagen, and three or four times as many operations -- is any less risky.)
The commentary on the piece, by Stan Grothos, notes some of this:
The neccessity for large quantities of bone morphogenetic protein-7 (BMP-7) requires more critical assessment, especially in relation to the impurity of the cellular component used...
Warnke and colleagues’ procedure needs greater numbers of patients and a more rigorous scientific assessment of the nature of the implants over a longer time to improve efficacy. In particular, the analysis of the bony tissue in the implants provides no direct evidence that a normal mandibular structure had been formed or whether there has been successful long-term integration into the surrounding tissue. However, Warnke's primary goal was to produce an implant that approximated the dimensions of a normal mandible, to provide successful functional and aesthetic outcomes for their patient. Although an anatomically correct mandible might not be produced by the present method, a structure resembling a normal mandible needs further testing of the functional loading capacity of the bony substitute.
This is a roundabout way of saying that nothing has been accomplished, other than proof-of-concept. And, like the UMass mouse photo above shows, the concept was proven pretty well eight years ago. Actually, the original Vacanti paper is more innovative than this new work -- at least their initial scaffold was flimsy and biodegradable, and the subsequent engineered tissue was found to be histologically similar to elastic cartilage.
So, despite advances in stem cell differentiation and cell attachment research, tissue engineering continues to be a shotgun-driven field. But hey, whatever works -- the man is chewing his food again. One colleague remarked, "I'm not sure what's more disturbing -- the fact that they grew a jaw in his back, or the fact that his first choice for a meal was a bratwurst sandwich."
I'm willing to venture: the most disturbing thing about this is that it's wasteful, dangerous, doesn't tell us anything new, and probably wouldn't haven been approved in this country.
